- +86 025-84430028、025-66113315
- sale05@cqherb.com
Your Location:Home >Products >Biochemical Engineering >84605-18-5

Appearance:Colorless needle crystal
Purity:99%
|
Description |
Cycloastragenol is a triterpenoid isolated from various legume species in the genus Astragalus that is purported to have telomerase activation activity. TA-65 is capable to increase average telomere length and to decrease the percentage of critically short telomeres and DNA damage in MEFs that harbor critically short telomeres. The effects on telomerase appear to be in common with other triterpenoid saponins (ie, ginsenosides RG1 and Rg3). These compounds at concentrations from 1 to 20 μM were reported to protect against IL-1β-, H2O2-, and tert-butylhydroperoxide-induced senescence in human chondrocytes, endothelial progenitor cells and fibroblasts, respectively. |
|
Uses |
Cycloastragenol is an aglycone derivative of astragaloside IV found in the root of Korean Astragalus membranaceus. |
|
Definition |
ChEBI: A sapogenin that is the aglycone derivative of astragaloside IV, a major saponin extracted from the root of Astragalus membranaceus. |
InChI:InChI=1/C30H50O5/c1-24(2)20(33)8-11-30-16-29(30)13-12-26(5)23(28(7)10-9-21(35-28)25(3,4)34)18(32)15-27(26,6)19(29)14-17(31)22(24)30/h17-23,31-34H,8-16H2,1-7H3/t17-,18-,19-,20-,21+,22?,23?,26+,27-,28-,29?,30?/m0/s1
The new cycloartane glycoside cyclochivi...
The new cycloartane glycoside cycloascau...
The invention belongs to the field of ph...
Methods and cosmetic compositions for co...
The structure of a new cycloartane trite...
-
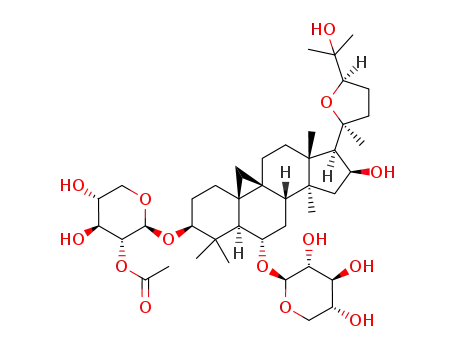
20S,24R-epoxycycloartan-3,6,16,25-tetraol-3-O-β-D-(2'-O-acetyl)xylopyranosyl-6-O-β-D-xylopyranoside

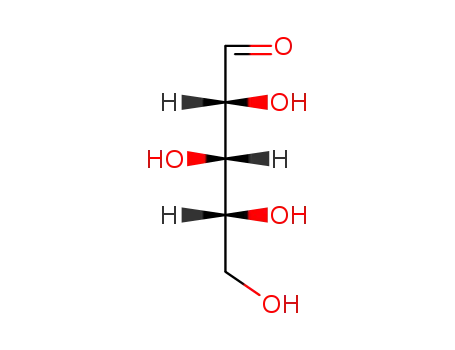
D-xylose

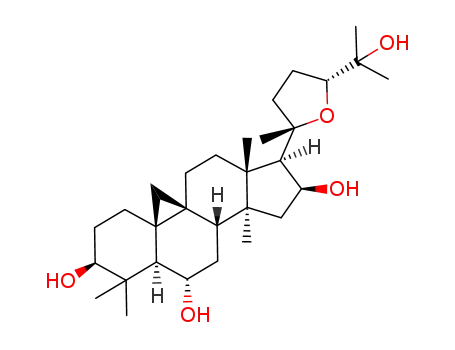
20(S),24(R)-epoxy-9β,19-cyclolanostan-3β,6α,16β,25-tetrol
| Conditions | Yield |
|---|---|
|
Acidic conditions;
|
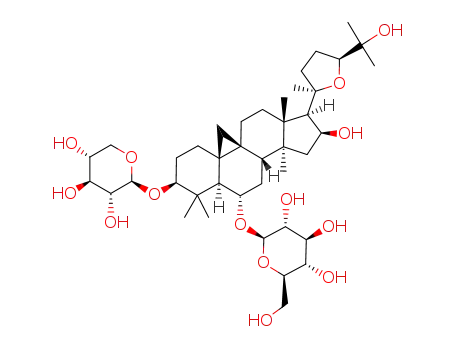
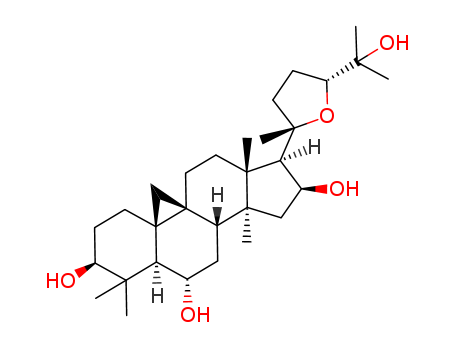
astragaloside IV

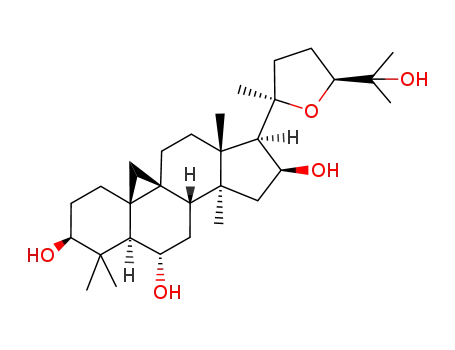
cycloastragenol
| Conditions | Yield |
|---|---|
|
With hydrogenchloride; In methanol; at 20 ℃; for 168h;
|
64% |
|
With hydrogenchloride; In methanol; at 20 ℃; for 168h;
|
64% |
|
With hydrogenchloride; In ethanol; benzene; for 24h; Heating;
|
30 mg |
|
With sulfuric acid; In methanol; for 3h;
|
30 mg |
|
Multi-step reaction with 2 steps
1: 47 mg / H2SO4 / methanol / 4 h / Heating
2: 17 mg / H2SO4 / methanol / 4 h / Heating
With sulfuric acid; In methanol;
|
|
|
Multi-step reaction with 2 steps
1: 58 mg / H2SO4 / methanol / 4 h / Heating
2: 11 mg / H2SO4 / methanol; H2O / 4 h / Heating
With sulfuric acid; In methanol; water;
|
|
|
With water; Acidic conditions;
|
|
|
astragaloside IV; With sodium periodate; In methanol; water; at 20 ℃;
With sodium tetrahydroborate; In methanol; at 20 ℃; for 24h;
With sulfuric acid; In methanol; water; for 24h; pH=2;
|
384 mg |
|
With hydrogenchloride; In methanol; chloroform; water; at 20 ℃; for 144h; Concentration;
|
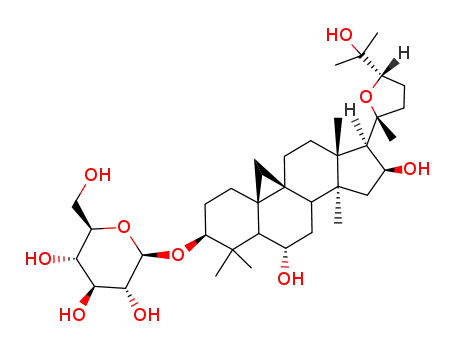
Cycloaraloside A
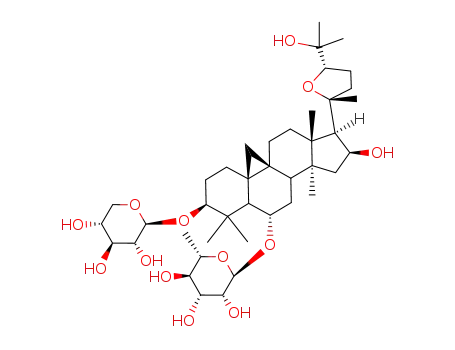
cyclocarposide
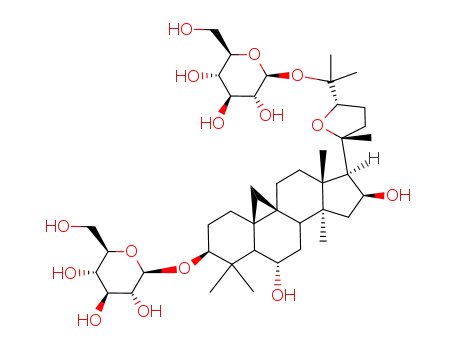
cycloaraloside E
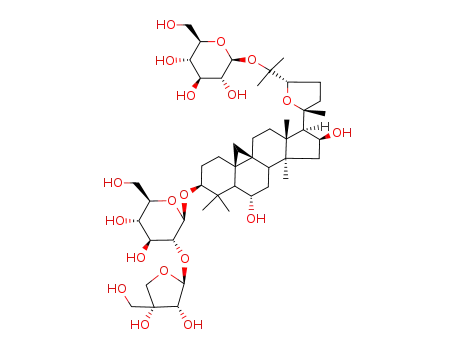
Cycloaraloside F
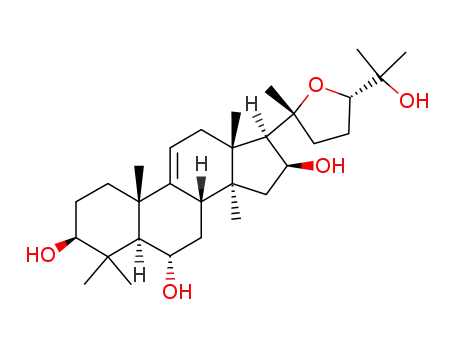
astragenol
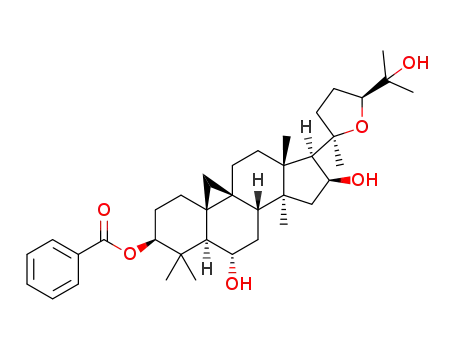
C37H54O6
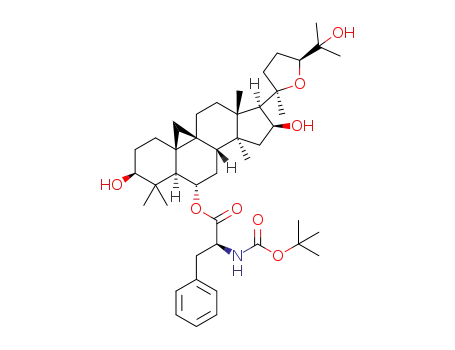
C44H67NO8
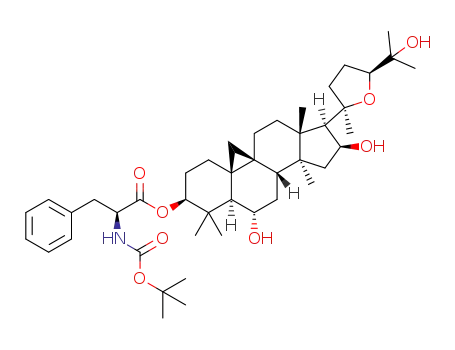
C44H67NO8
CAS:82373-94-2
CAS:83207-58-3
CAS:16837-52-8
CAS:63223-86-9